The Microbe-Host Interface: Molecular Mechanisms Mediating Protective and Pathological Innate and Adaptive Immune Responses within the Gut - Swiss National Science Foundation - Sinergia
In the healthy subject, host and gut microbiota live in a mutualistic relationship. The gut microbiota provides both protective and damaging signals to the intestinal immune system, which under homeostatic conditions are well tolerated by the host. In intestinal inflammation such as Inflammatory Bowel Disease (IBD), the host-microbiota homeostasis is disturbed leading to chronic inflammation. IBD impacts the life of 12’000 to 15’000 people in Switzerland alone. Although the exact etiology of IBD remains unclear, several studies suggest multifactorial cause encompassing genetic predisposition, environmental factors and the intestinal microbiota. To date, IBD lacks a satisfactory curative treatment adaptable to the majority of patients.
Our central hypothesis is that a dysbiotic microbial community is a key determinant in increasing the risk of colitis development, but on the other hand, reestablishing a ‘healthy’ microbial community is able to reverse the inflamed state of the intestine. The overall goal of this project is therefore to elucidate molecular mechanisms that are fundamental to host-microbiota interactions in acute colitis and design rationale microbial-based intervention strategies for the treatment of IBD.
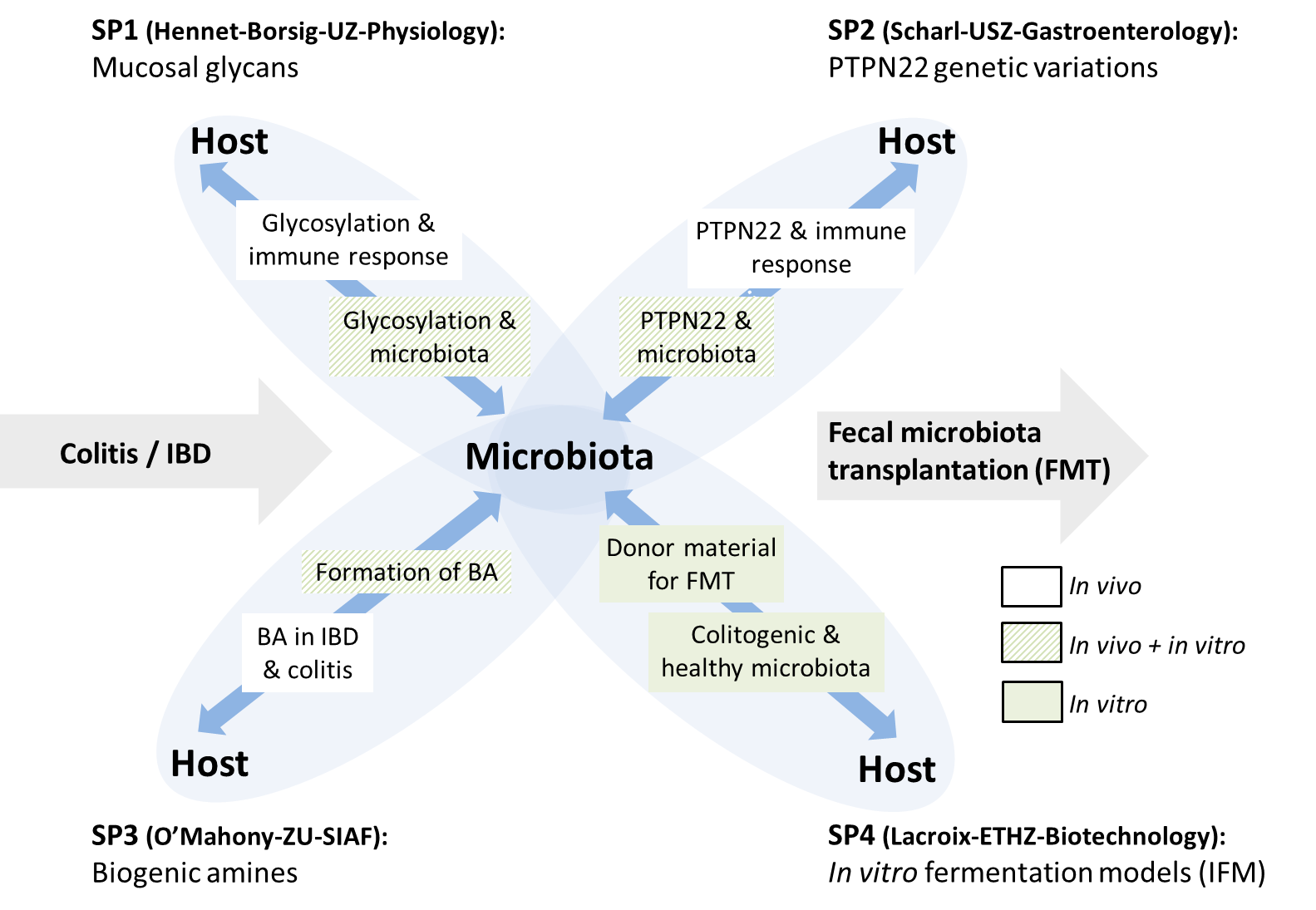
This synergistic project regroups researchers from the fields of immunology, microbiology and biotechnology, gastroenterology and glycobiology from different Swiss universities, with unique expertise in both basic and clinical research. Advanced in vivo and in vitro approaches and state-of-the art methods in immunology, microbiology, and glycobiomics are used for the following objectives:
- Study the effects of intestinal microbiota on immune responses at the intestinal surface using well-established cell lines, primary human cells, colitis and knock-out mouse models;
- Investigate the role of specific complex chemically synthesized carbohydrates in regulating mucosal immunity and the role of their sialylation and fucosylation;
- Unravel the role of biogenic amine levels on the host immune system in IBD;
- Assess how genetic variations and altered physiological conditions affect microbiota composition in vitro and in vivo;
- Validate advanced in vitro intestinal fermentation models for controlled production of functional and safe intestinal microbiota for microbiota transplantation for the treatment of IBD.
Within this project, the composition and the effects of fecal microbiota derived from genetically susceptible and non-susceptible hosts involving the Swiss IBD Cohort with its about 2’500 patients on the development of colitis and the regulation of innate immune responses are investigated. We apply mouse models that are impacted in epithelial barrier function due to variations in regulatory networks or mucus layer structure and composition (PTPN22-/-, Muc2-/-, Fut2-/-) and determine the impact of those modifications to colitis susceptibility and microbiota composition and activity in vivo. We concurrently investigate the consequence of shifts in microbial community composition and metabolite formation due to changes in substrate availability on immune response in vitro. All data from the different sub-projects are integrated to provide a detailed overview of the state of host and microbiota in IBD. This knowledge is used to develop and validate novel therapeutic treatments for IBD patients based on transplantation of in vitro produced functional intestinal microbiota.